microencapsulation drug delivery
/Advancing Pharmaceutical & Nutraceutical Delivery: Industry Trends in Controlled Release Technologies The demand for sophisticated drug and nutrient delivery systems continues to grow exponentially, driven by a global focus on enhanced therapeutic efficacy, improved patient compliance, and reduced side effects. Within this evolving landscape, controlled release technologies, particularly in tablet formulations, represent a critical frontier. These innovative systems enable active pharmaceutical ingredients (APIs) and nutraceutical compounds to be released over an extended period, maintaining stable plasma concentrations and optimizing desired physiological effects. Key industry trends underscore a shift towards personalized medicine and functional nutrition, where precise dosing and prolonged action are paramount. This necessitates robust OEM/ODM partnerships capable of developing bespoke formulations. The global controlled-release drug delivery systems market, valued at approximately $60 billion in 2022, is projected to reach over $100 billion by 2030, reflecting a CAGR of around 7.0%. This growth is propelled by an aging population, rising prevalence of chronic diseases, and technological advancements in polymer science and formulation techniques. As a result, the strategic importance of reliable Time Release Tablet OEM/ODM Premix Service providers has never been higher, offering the expertise and infrastructure to bring complex formulations to market efficiently and compliantly. Innovations in matrix systems, osmotic pumps, and multi-layered tablets are pushing the boundaries of what's achievable in sustained, pulsatile, and targeted release profiles. This ensures that the active compounds are delivered exactly where and when they are needed, enhancing both safety and effectiveness. Comprehensive Process Flow for Time Release Tablet Manufacturing The development and manufacturing of controlled-release tablets require a meticulous, multi-stage process to ensure precise dosage, consistent release profiles, and optimal stability. Our approach to providing a Time Release Tablet OEM/ODM Premix Service integrates advanced scientific principles with rigorous quality control at every juncture. 1. Conceptualization & Premix Formulation This initial phase involves extensive R&D, client consultation, and active ingredient compatibility studies. We leverage our expertise in polymer science and excipient functionality to design the optimal premix. Key considerations include the desired release kinetics (e.g., zero-order, first-order, pulsatile), active pharmaceutical ingredient (API) or nutraceutical solubility, stability, and target dissolution profile. Material selection typically includes hydrophilic polymers (e.g., HPMC, Carbopol), lipophilic matrices (e.g., waxes, fatty acids), pH-sensitive polymers, and osmotic agents. All raw materials are sourced from validated suppliers and undergo stringent quality checks to meet pharmacopeial standards (USP, EP, JP). 2. Granulation / Direct Compression Depending on the formulation, ingredients may undergo wet granulation, dry granulation, or direct compression. Wet granulation enhances flowability and compressibility, crucial for uniform tablet weight and strength. Dry granulation (roller compaction) is preferred for moisture-sensitive APIs. Direct compression, when feasible, minimizes processing steps. This stage is critical for ensuring content uniformity and reproducible tablet characteristics. Equipment used includes high-shear granulators, fluid bed processors, and roller compactors. 3. Tablet Compression The granulated or directly compressible blend is fed into high-speed tablet presses. Parameters such as compression force, dwell time, and punch/die specifications are precisely controlled to achieve target hardness, friability, thickness, and weight. Uniformity of these physical parameters is continuously monitored using in-process controls to ensure batch consistency and mechanical integrity throughout the product's service life. 4. Coating (Film or Enteric) Many time-release tablets benefit from a coating. Film coatings improve swallowability, mask taste, and protect the tablet core from moisture or light degradation. Enteric coatings are applied to prevent premature drug release in the acidic stomach environment, ensuring dissolution in the higher pH of the small intestine. This is crucial for APIs that are acid-labile or can cause gastric irritation. Coating processes are conducted in perforated coating pans with precise control over spray rate, air temperature, and pan speed. 5. Quality Control & Testing Each batch undergoes rigorous quality assurance and quality control testing, adhering to international standards such as ISO 9001 and GMP (Good Manufacturing Practices) guidelines. Key tests include: Dissolution Testing: Critical for controlled release products, evaluating the rate and extent of API release over time under simulated physiological conditions (e.g., USP Apparatus 1 & 2). Assay: Quantifying the active ingredient content to ensure potency. Content Uniformity: Verifying consistent API distribution across individual tablets. Hardness & Friability: Assessing mechanical strength to withstand handling and packaging. Disintegration Time: For immediate-release layers or to confirm coating integrity. Stability Studies: Accelerating and real-time studies (ICH guidelines) to determine shelf-life under various temperature and humidity conditions. Microbiological Purity: Ensuring absence of harmful microorganisms. 6. Packaging & Labeling Finished tablets are carefully packaged into blisters, bottles, or sachets, utilizing appropriate materials that protect against moisture, light, and oxygen, thereby extending product shelf life and maintaining efficacy. Precise labeling ensures compliance with regulatory requirements and provides clear product information to end-users. Technical Specifications and Controlled Release Mechanisms Understanding the underlying technical specifications and release mechanisms is fundamental to appreciating the sophistication of a Time Release Tablet OEM/ODM Premix Service . Our formulations are engineered to precise pharmacokinetic and pharmacodynamic profiles. Controlled Release Mechanisms Diffusion-Controlled Systems: The API permeates through an insoluble matrix or membrane coating. Release rate is governed by Fick's Law of Diffusion, dependent on concentration gradient and membrane permeability. Dissolution-Controlled Systems: The API is encapsulated within a slowly dissolving matrix or coated with a dissolving polymer. Release is governed by the rate of dissolution of the matrix or coating. Erosion-Controlled Systems: The tablet matrix slowly erodes or degrades in the physiological environment, releasing the API as it breaks down. Osmotic Systems: These sophisticated systems utilize osmotic pressure to deliver the API at a constant rate. A semi-permeable membrane surrounds the drug core, and as water enters, it creates pressure that pumps the API out through a laser-drilled orifice. Ion-Exchange Resins: APIs complexed with ion-exchange resins are released by exchange with ions present in the gastrointestinal tract. Typical Time Release Tablet Specifications Parameter Specification Range Method/Standard Active Ingredient Load 5 mg - 1000 mg (customizable) HPLC, UV-Vis Spectrophotometry Release Duration 4 hours to 24 hours (customizable) USP Dissolution Apparatus 1 & 2 Tablet Hardness 50 N - 250 N (typically 80-150 N) Pharmacopoeial hardness tester Friability Typically < 1.0% loss in weight USP Friability Tester Disintegration Time (for specific layers/coatings) As per targeted profile (e.g., immediate release layer < 15 min) USP Disintegration Apparatus Content Uniformity RSD < 6.0% (individual tablets) USP Chapter <905> Stability Data (Accelerated) 3 months at 40°C/75% RH, <5% degradation ICH Guidelines Q1A(R2) These specifications are customized for each project, ensuring that the final product meets the exact requirements for efficacy, safety, and regulatory compliance. The precision involved in achieving these metrics highlights the expertise required in a high-quality Time Release Tablet OEM/ODM Premix Service . Application Scenarios and Strategic Advantages The versatility of controlled-release formulations makes them indispensable across a wide spectrum of industries. A proficient Time Release Tablet OEM/ODM Premix Service offers solutions that translate directly into significant advantages for both manufacturers and end-users. Key Application Industries: Pharmaceuticals: Treating chronic conditions such as hypertension, diabetes, depression, and pain management. Sustained release reduces peak-and-trough plasma fluctuations, minimizing side effects and improving therapeutic outcomes. Example: Metformin extended-release for Type 2 Diabetes. Nutraceuticals & Dietary Supplements: Delivering vitamins, minerals, herbal extracts, and probiotics with optimized absorption and prolonged action. This prevents rapid excretion and maximizes the physiological benefits. Example: Multivitamin formulations for continuous nutrient supply throughout the day. Sports Nutrition: Sustained release of amino acids, energy compounds, or performance enhancers to support muscle recovery and sustained energy levels over extended periods. Example: Branched-Chain Amino Acid (BCAA) tablets for consistent muscle support. Veterinary Medicine: Providing long-acting treatments for animals, improving compliance and reducing handler stress associated with frequent dosing. Strategic Advantages in Typical Scenarios: Enhanced Bioavailability and Efficacy: By controlling the release rate, APIs are delivered to the optimal absorption window, maximizing the amount of drug that reaches systemic circulation and enhancing its therapeutic effect. For example, in extended-release formulations, the API is released slowly, avoiding the "first-pass effect" and maintaining steady concentrations. Improved Patient/Consumer Compliance: Reduced dosing frequency (e.g., once daily instead of multiple times) significantly improves adherence, especially for chronic therapies. This translates to better health outcomes and higher customer satisfaction. Reduced Side Effects: Avoiding high peak plasma concentrations minimizes dose-related adverse effects, leading to a safer and more tolerable product profile. This is crucial for potent APIs or those with a narrow therapeutic index. Optimized Resource Utilization: For manufacturers, the ability to outsource complex time-release tablet production via an OEM/ODM service allows for focusing on core competencies like R&D and marketing, without significant capital expenditure on specialized manufacturing equipment. Corrosion Resistance (for certain matrix components): While not directly "corrosion resistance" in the traditional sense, the carefully chosen excipients and coating materials in our formulations are designed to withstand various gastrointestinal environments, ensuring the active ingredient is released only at the intended site and time, protecting it from degradation by gastric acids. Energy Saving (indirectly): By improving patient compliance and therapeutic outcomes, these formulations can indirectly contribute to energy savings in healthcare systems by reducing hospital visits and managing chronic conditions more effectively. Technical Advantages and Vendor Comparison Selecting the right OEM/ODM partner for Time Release Tablet OEM/ODM Premix Service is a critical decision. Our distinct technical advantages ensure superior product quality, reliability, and market competitiveness. Our Technical Advantages: Precision Release Profile Engineering: Utilizing advanced modeling and simulation tools, we precisely design release kinetics for sustained, pulsatile, or multi-phasic delivery, tailored to specific APIs and therapeutic needs. This includes multi-layered tablet technology for varied release stages. Advanced Polymer & Excipient Expertise: Deep knowledge in selecting and combining functional excipients (e.g., controlled-release polymers like HPMC, ethyl cellulose, PEO) to achieve desired drug release mechanisms, tablet integrity, and stability across diverse environmental conditions. Robust Stability & Shelf-Life: Formulations are optimized not only for release characteristics but also for chemical and physical stability, ensuring long shelf-life under various storage conditions, minimizing degradation of active ingredients. Scalability and Manufacturing Efficiency: Our processes are designed for seamless scale-up from pilot to commercial batches, maintaining consistent product quality and optimizing manufacturing costs without compromising integrity. Comprehensive Analytical Capabilities: State-of-the-art analytical labs conduct in-depth characterization, including dissolution testing across multiple pH media, advanced chromatographic techniques for purity, and particle size analysis, ensuring every batch meets stringent quality standards. Regulatory Compliance Expertise: Our processes and documentation adhere strictly to international regulatory requirements (e.g., FDA, EMA, Health Canada, TGA), facilitating faster market entry for client products. Vendor Comparison: Finutra vs. Generic Providers Feature/Metric Finutra's Offering Generic/Less Specialized Provider R&D and Formulation Expertise Dedicated team, advanced modeling, bespoke polymer selection, multi-release profile design. Standard templates, limited customization, basic polymer choices. Quality Control & Testing Comprehensive in-process and finished product testing, ICH-compliant stability, full analytical suite. Basic pharmacopoeial tests, limited advanced characterization. Customization Capability High degree of customization for API load, release profile, dosage form, excipients, and coating. Limited options, primarily off-the-shelf solutions. Regulatory Support Expert guidance, compliant documentation, audit readiness, global market access support. Basic documentation, client largely responsible for regulatory affairs. Lead Time & Efficiency Optimized processes, efficient supply chain management, realistic timelines. Variable, potentially longer due to less integrated systems. IP Protection Robust confidentiality agreements and secure data management protocols. Less stringent or ambiguous IP clauses. This comparison highlights Finutra's commitment to delivering a premium Time Release Tablet OEM/ODM Premix Service , providing a clear competitive edge through superior scientific rigor and operational excellence. Customized Solutions: Tailoring Your Time Release Formulations The strength of an exceptional Time Release Tablet OEM/ODM Premix Service lies in its ability to offer unparalleled customization. We understand that each active ingredient, therapeutic objective, and market demand presents unique challenges. Our approach is collaborative, transforming your specific requirements into a high-performance, market-ready product. Key Areas of Customization: API/Ingredient Selection and Concentration: We work with a vast array of APIs and nutraceutical compounds, from highly soluble to poorly soluble. Our formulation experts will optimize concentration to achieve the desired therapeutic dose within the specified release window. Release Profile Design: Whether you require a sustained, extended, pulsatile, or targeted release, we can engineer the specific release kinetics. This involves manipulating matrix properties, coating thickness, and excipient combinations to achieve the precise in-vitro and in-vivo dissolution characteristics. Excipient Optimization: Selection of rate-controlling polymers, binders, diluents, disintegrants, and lubricants is critical. We custom-select excipients to ensure compatibility, enhance stability, and achieve desired physical tablet properties (hardness, friability). Dosage Form and Size: Tablets can be customized in terms of size, shape (e.g., round, oval, caplet), and scoring for patient convenience or adjustable dosing. Coating Options: From functional enteric coatings for pH-dependent release to aesthetic film coatings for improved swallowability, taste masking, and brand recognition, our coating specialists offer diverse solutions. Flavoring and Coloring: For nutraceuticals or pediatric applications, taste and visual appeal can be crucial. We offer various flavoring and coloring agents that do not interfere with the active ingredient or the release mechanism. Packaging Solutions: We provide tailored primary and secondary packaging options, including blister packs, bottles, pouches, and customized branding, ensuring product integrity and market appeal. Our collaborative development process involves rigorous prototyping, testing, and feedback loops to ensure the final product aligns perfectly with your vision and regulatory requirements. This iterative approach minimizes risks and accelerates time-to-market. Application Case Studies & Client Success Our experience in providing a robust Time Release Tablet OEM/ODM Premix Service is demonstrated through successful partnerships and innovative product launches. These case studies highlight our capability to deliver complex formulations with measurable impact. Case Study 1: Sustained-Release Vitamin B Complex Client Challenge: A prominent nutraceutical brand sought to develop a Vitamin B complex tablet that would release nutrients gradually throughout the day, avoiding the "flush effect" and ensuring sustained energy. Existing products on the market provided a rapid burst, leading to inefficient absorption and customer dissatisfaction. Our Solution: We developed a multi-layered tablet utilizing a hydrophilic matrix for controlled diffusion and a pH-dependent enteric coating for specific B vitamins sensitive to gastric acidity. The premix formulation incorporated specific grades of HPMC and PEO, carefully balanced to achieve a 12-hour sustained release profile. Outcome: Dissolution studies confirmed a consistent release of B vitamins over 12 hours. Pilot bioavailability studies demonstrated significantly improved nutrient absorption and reduced peak concentrations, minimizing side effects. The client successfully launched the product, receiving positive customer feedback on sustained energy and absence of adverse reactions, leading to a 30% market share increase in the premium vitamin segment within the first year. Case Study 2: Once-Daily Pharmaceutical for Chronic Pain Client Challenge: A pharmaceutical company needed a once-daily extended-release formulation for an opioid analgesic to reduce dosing frequency and improve patient compliance for chronic pain management, while also mitigating potential for abuse by avoiding rapid drug release. Our Solution: We engineered an osmotic pump tablet system with a specialized semi-permeable membrane and a laser-drilled orifice. The core formulation included the active ingredient, osmotic agents, and a push layer. Rigorous in-vitro dissolution testing was performed at various pH levels to mimic GI transit, ensuring a true zero-order release over 24 hours. Outcome: The resulting tablets demonstrated excellent in-vitro/in-vivo correlation, maintaining stable plasma concentrations of the analgesic for a full 24-hour period. Clinical trials showed significant improvements in patient compliance and reduced incidence of adverse events compared to immediate-release counterparts. The product received regulatory approval and became a flagship offering for the client, revolutionizing pain management for many patients. Customer Feedback: "The expertise and attention to detail provided by Finutra's Time Release Tablet OEM/ODM Premix Service were instrumental in bringing our innovative supplement to market. Their scientific rigor in formulation development and unwavering commitment to quality surpassed our expectations." - Head of Product Development, Leading Health Supplement Brand. "Partnering with Finutra on our extended-release drug candidate was a strategic decision. Their deep technical knowledge and seamless process flow ensured a compliant, high-quality product that significantly improved patient outcomes." - VP of Pharmaceutical Operations, Biotech Innovator. Authoritativeness and Trustworthiness: Our Commitment Our reputation as a leading provider of Time Release Tablet OEM/ODM Premix Service is built on a foundation of unyielding quality, adherence to international standards, and transparent operational practices. We are committed to fostering trust through our certifications, client partnerships, and comprehensive support services. Certifications & Compliance: ISO 9001:2015 Certified: Demonstrating our commitment to a robust Quality Management System across all operations. GMP (Good Manufacturing Practices) Compliant: Our facilities and processes adhere to strict international GMP guidelines, ensuring products are consistently produced and controlled according to quality standards appropriate to their intended use and as required by the marketing authorization. FDA Registered (where applicable for specific products/markets): Operating in compliance with relevant FDA regulations for dietary supplements and pharmaceutical components. Halal/Kosher Certified (upon request): Providing flexibility to meet diverse market and consumer demands. Partnerships & Experience: With over a decade of dedicated service in the pharmaceutical and nutraceutical sectors, we have cultivated strong relationships with numerous global and regional brand leaders. Our portfolio includes successful collaborations with companies spanning emerging biotech startups to established multinational corporations, consistently delivering innovative and high-quality controlled-release solutions. Frequently Asked Questions (FAQ): Q: What is the typical lead time for a new custom time-release tablet project? A: Lead times vary based on project complexity and formulation requirements. Generally, from initial consultation to pilot batch production, it ranges from 12-24 weeks, followed by 4-8 weeks for commercial scale-up and validation. Specific timelines are provided after a detailed project scope review. Q: Do you provide stability data for your formulations? A: Yes, we conduct comprehensive stability studies in accordance with ICH guidelines (accelerated and real-time) to determine product shelf-life and optimal storage conditions. Full stability reports are provided as part of our service. Q: What are your minimum order quantities (MOQs) for OEM/ODM services? A: MOQs are flexible and depend on the specific product and manufacturing process. We strive to accommodate clients of all sizes and will discuss MOQs during the initial project assessment to find a mutually beneficial arrangement. Q: How do you ensure intellectual property (IP) protection for client formulations? A: We operate under strict confidentiality agreements (NDAs) and have robust internal protocols for IP protection. Your formulation and proprietary information are handled with the utmost security and discretion. Warranty & Customer Support: We stand by the quality of our products and services. All manufactured products come with a warranty covering manufacturing defects and compliance with agreed-upon specifications. Our dedicated technical support team is available to assist with any post-delivery inquiries, troubleshooting, or additional support required. We aim for long-term partnerships built on trust and continuous support. Conclusion The landscape of pharmaceutical and nutraceutical delivery is continuously shaped by the need for advanced, patient-centric solutions. Our specialized Time Release Tablet OEM/ODM Premix Service stands at the forefront of this evolution, offering unmatched expertise in formulation development, manufacturing precision, and rigorous quality assurance. By partnering with us, clients gain access to cutting-edge technology, comprehensive regulatory compliance, and a collaborative approach designed to bring innovative controlled-release products to market efficiently and successfully. We are committed to empowering your brand with products that deliver superior therapeutic outcomes, enhance patient compliance, and ensure market differentiation. References Lachman, L., Lieberman, H. A., & Kanig, J. L. (1987). The Theory and Practice of Industrial Pharmacy (3rd ed.). Lea & Febiger. Remington: The Science and Practice of Pharmacy (23rd ed.). (2020). Pharmaceutical Press. United States Pharmacopeia (USP) and National Formulary (NF). Current Edition. International Conference on Harmonisation (ICH) Guidelines Q1A(R2) - Stability Testing of New Drug Substances and Products. Khan, S., & Akhlaq, M. (2014). Overview of Controlled Release Oral Drug Delivery Systems. Journal of Pharmacy and Allied Health Sciences, 4(1).
Finutra devotes to be an integrated supplier for global supply chain, we offer a
broad array of raw materials and functional ingredients
Authoritative Certification
Continuous Innovation, Customer First
Enhance core competitiveness to bring customers better products and services,
Each of these is the result of our team's relentless pursuit of excellence
and our deep commitment to social responsibility.








Global
Reach
FINUTRA has over 350,000 square feet of manufacturing and warehouse
space worldwide.
Industries We Serve
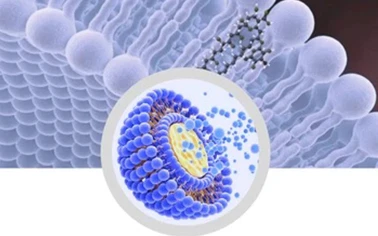
Advanced molecular distillation and microencapsulation
technology. Extremely bioavailable
trace carotenoids Intuitively soluble.


STAY UPDATED
Receive special offers and first look at new
products.
products.
Building 23B1, No.2 Yuanboyuan St., Zhengding Area of China (Hebei) Pilot Free Trade Zone
QUICK LINK
Finutra devotes to be an integrated supplier for global supply chain, we offer a broad array of raw
materials and functional ingredients as a manufacturer, distributor and supplier for global Beverage,
Nutraceutical, Food, Feed and Cosmeceutical.
Copyright © 2025 Hebei Finutra
Biotech Co.,
Ltd. All
Rights Reserved.
Privacy Policy